Abstract
Introduction: Positron emission tomography-computed tomography (PET-CT) has been utilized in CLL to help identify patients (pts) with Richter's transformation (RT). Data from kinase inhibitor (KI)-naïve pts suggest that maximum standardized uptake value (SUVmax) of ≥10 on PET can distinguish RT from CLL with 91% sensitivity, 95% specificity (Michallet, 2014). Additionally, in the setting of CLL progression, SUVmax ≥10 identified pts with inferior survival, independent of RT diagnosis (Falchi, 2014). Whether PET can accurately identify and risk-stratify pts failing KI therapy with RT vs. CLL is unknown. In a phase 2 trial (NCT021412820), treatment with the BCL-2 inhibitor venetoclax (VEN) was evaluated in pts in whom prior ibrutinib or idelalisib had failed. All consented pts were required to undergo PET imaging to identify and exclude RT. Therefore, this represents the largest, uniform dataset of KI-treated pts who have undergone PET imaging and were subsequently uniformly treated.
Methods: Pts were evaluated at screening by PET and excluded from the study if RT was confirmed on core biopsy of the most suspicious node. Biopsy of the suspicious area was mandatory if PET SUVmax was ≥10 or for pts with CD38+, ZAP 70+, TP 53 mutated and IGHV unmutated CLL with an SUVmax 4-10 with ≥1 of the following: B symptoms, nodes >5 cm, and/or LDH elevation. We used descriptive methods, logistic regression, and receiver operator characteristic (ROC) analysis to define post-KI PET test characteristics.
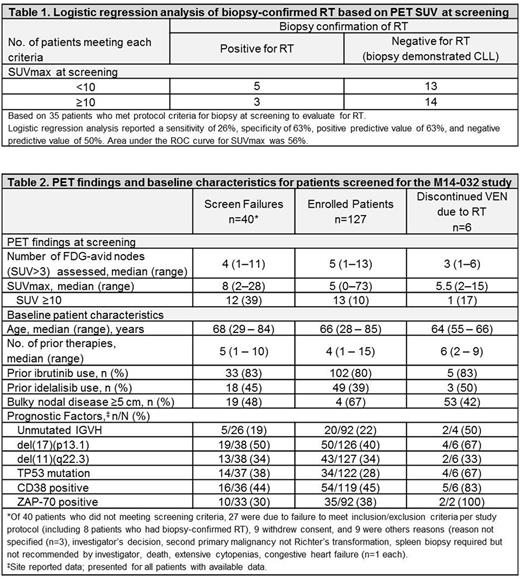
Results: 167 pts were screened, of whom 84 (50%) had PET SUV ≥5 and 25 (15%) had SUV ≥10. 57 pts met protocol criteria for biopsy at screening to evaluate for RT (SUV ≥10, n=18; SUV <10 with other factors, n=17): 35 underwent biopsy and 22 did not meet enrollment criteria for other reasons so no biopsy was performed. Of these 35 pts, 8 had RT (failed screening due to confirmed RT [all with large B-cell lymphoma]), 2 had other malignancies (metastatic anal cancer and neuroendocrine tumor), and 25 had biopsy demonstrating CLL. Based on a logistic regression analysis of 35 pts who underwent biopsy (Table 1), PET SUVmax ≥10 did not adequately distinguish RT post-KI therapy vs. CLL (OR, 1.79 [95% CI: 0.4-9]; p=.5) with 26% sensitivity, 82% specificity, 63% positive predictive value, and 50% negative predictive value. ROC area for SUVmax ≥10 was 56%.
For pts who failed screening, median number of FDG-avid nodes with an SUV >3 was 4 (range: 1-11) and median SUVmax was 8 (range: 2-28), with 12 (39%) who had SUV ≥10 (Table 2). For 127 enrolled pts, median number of FDG-avid nodes was 5 (range: 1-13) and median SUVmax was 5 (range: 0-73), with 13 (10%) who had SUV ≥10. There was a statistical trend for higher SUVmax for pts who did not meet screening criteria vs enrolled (p=.0607). The objective response rate to VEN was similar for pts when stratified by screening PET SUV ≥10 (62%, 8/13) vs <10 (56%, 53/94). Median PFS was also similar for pts when stratified by screening PET SUV ≥10 (19.2 months [95% CI: 0.4, -]) vs <10 (21.9 months [95% CI: 15.9, -]).
Fifty-six (44%) pts discontinued VEN, with 28 (22%) due to CLL progression and 6 (5%) due to biopsy-confirmed RT following both imaging and clinical changes. Median time to CLL progression was 8.4 months (range: .1-22.8) and to RT was 11.5 months (range: 4.4-19.7). For pts who discontinued VEN due to RT, median number of FDG-avid nodes at screening (pre-VEN) was 3 (range: 1-6) and SUVmax was 5.5 (range: 2-15). PET SUV ≥10 at screening did not predict development of subsequent RT when on VEN (OR, 1.483 [95% CI: .2-13.8]; p=.7289). Compared with 127 enrolled pts, pts who developed RT while on VEN were older, had a higher median number of prior therapies, and the majority had at least 1 poor-risk prognostic feature at study enrollment (Table 2).
Conclusions: In the largest series of PET-CTs prospectively performed in pts following KI discontinuation, we conclude that PET SUV ≥10 alone lacks both sensitivity and specificity to distinguish CLL progression vs RT. CLL progression following KI exposure appears more metabolically active than previously reported following R-chemotherapy failure. RT was confirmed for 14% (8/57) of pts suspected of CLL progression with BCRi therapy, which suggests that screening for RT may be important upon BCRi failure. In addition, PET SUV ≥10 did not identify VEN-treated patients with an inferior ORR or PFS. Analysis to identify clinical factors distinguishing KI-treated pts with likely RT is ongoing.
Mato: TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; DTRM: Research Funding; AbbVie: Consultancy, Research Funding; Portola: Research Funding; Kite: Consultancy; Pharmacyclics: Research Funding; Janssen: Consultancy; AstraZeneca: Consultancy; Regeneron: Research Funding; Acerta: Research Funding; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Wierda: Pharmacyclics: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Acerta: Research Funding; GSK/Novartis: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy, Honoraria; Kite: Research Funding; Janssen: Research Funding; Sanofi: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Genentech/Roche: Consultancy, Honoraria, Research Funding; Emergent: Consultancy, Honoraria, Research Funding; Karyopharm: Research Funding; Juno: Research Funding; Merck: Consultancy, Honoraria; The University of Texas MD Anderson Cancer Center: Employment. Davids: Celgene: Consultancy; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy; Infinity: Consultancy, Research Funding; InCyte: Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cheson: AbbVie, Roche-Genentech, Pharmacyclics, Acerta: Consultancy; Acerta, Pharmacyclics, Epizyme, Gilead, Roche, AbbVi: Other: Institution receives research support . Coutre: AbbVie: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Janssen: Consultancy; Novartis: Consultancy, Research Funding; Gilead: Consultancy, Research Funding. Choi: AbbVie, Genentech, and PCYC: Consultancy; AbbVie: Other: Institutional research funding; Gilead, Genentech, Abbvie, and PCYC: Speakers Bureau. Furman: AbbVie, Pharmacyclics, Janssen, Gilead, Genentech, Sunesis, Verastem: Consultancy. Heffner: ADC Therapeutics: Research Funding; Kite: Research Funding. Barr: Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Infinity: Consultancy; Celgene: Consultancy; Gilead: Consultancy; AbbVie: Consultancy, Research Funding; Novartis: Consultancy; Seattle Genetics: Consultancy. Eradat: AbbVie, Gilead, Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Speaker. Zhou: Abbvie: Employment, Equity Ownership. Verdugo: AbbVie: Employment, Equity Ownership. Potluri: Abbvie: Employment, Research Funding. Jones: Abbvie, Pharmacyclics, Genentech, Gilead, Janssen, Merck, and Acerta: Other: Institutional research funding; Sunesis: Other: Institutional research funding; Genentech, Abbvie, Pharmacyclics, Gilead, Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal